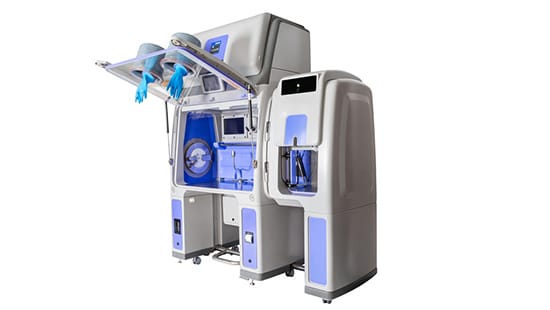
The Importance of Protecting Your Sterility Testing Process
Product samples are taken throughout the production process and tested for microbial contamination. In most cases the sterility test will demonstrate that the product is free of contamination, allowing the product to be dispatched. When the results show a contaminated product, delivery is halted to prevent exposing potential risk to patients.
If your sterility process is not suitably protected then contamination from the environment can impact the test and indicate that the product is contaminated when it is not. This can result in significant costs to the manufacturer, and potentially drug shortages to patients if the batch has to be scrapped. Help protect the sterility testing process from contamination in the surrounding environment to reduce your risk of a false positive.
Whether your methodology is membrane filtration or direct inoculation, an isolator can provide distinct advantages.

Discover Our Sterility Testing Isolator
Ecolab’s Bioquell Qube provides an aseptic ISO 5 / Grade A environment and includes integrated Bioquell Hydrogen Peroxide Vapour* technology, achieving a validated 6-log sporicidal kill on exposed surfaces within operating areas. From its polymer molding to its integration with the Merck Millipore Symbio Flex Sterility Pump, the Bioquell Qube is truly unique and an ideal solution for sterility testing.
*When used according to the label instructions, Ecolab Bioquell systems utilize Bioquell Hydrogen Peroxide Sterilant-AQ (EPA Registration Number:1677-277)
Is an isolator the right choice for you? Do you have the right isolator?
What would a false positive or issue mean for you?
Halting production due to a false positive could lead to significant downtime, unnecessary scrapping of product, drug shortages and potentially reduce a company’s standing in the marketplace. Plus the additional time, labour, and costs from CAPA related steps would need to be accounted for.
How much cleanroom space does the process require?
Cleanroom space typically needs to be optimised as pricing per square feet to build and operate the space can be quite costly. An isolator like the Bioquell Qube offers a small footprint that can be even be moved as needed easily, requires no construction, and operate in lower grade areas. For specifications, please contact Ecolab.
Does your sterility testing process require a Grade B cleanroom?
With isolators, you can perform testing in lower grade cleanrooms that require less or no gowning. Additionally, valuable cleanroom space can be conserved for other critical operations and can help reduce operating costs as higher grade cleanrooms require greater financial investments from energy and maintenance costs.
Is your current process validated and truly repeatable?
Automated bio-decontamination of incoming materials is a validated process, guaranteeing the same efficacy on every cycle. This further helps to decrease the risk of a false positive as a result.
How many sterility tests do you need to perform in one day?
Cleanroom space typically needs to be optimised as pricing per square feet to build and operate the space can be quite costly. An isolator like the Bioquell Qube offers a small footprint that can be even be moved as needed easily, requires no construction, and operate in lower grade areas.The Bioquell Qube, for example, can handle up to 60 tests per day depending on configuration. For specifications, please contact Ecolab.
Are you outsourcing your sterility testing process?
There are advantages to bringing the process in-house including more control over the process, long-term cost savings, and faster results. Selecting an isolator that can be hosted in CNC areas or lower grade cleanrooms allows this to become a reality for companies despite an upfront equipment related cost.

The Impact of Failure
The impact of a test failure can be significant for a pharmaceutical manufacturer as it typically results in:
- Withholding release and potentially scrapping the batch of product which failed the test
- Additional cleaning and disinfection of production areas
- Regulatory involvement
- Financial losses to the manufacture
- Preforming timely investigations
- Drug shortages to patients
- Halting of production of further product whilst investigations are preformed
However, sterility test failures are not always caused by contamination in the product.
A false positive can result when contamination from the environment or operator performing the test finds its way into the test, causing a failure. A product will be deemed as contaminated when in reality it is free of harmful microorganisms.
In the case of a sterility test failure, the burden of proof is on the manufacturer to demonstrate that the failure is the result of a contamination from the operator and/or lab environment, a difficult task to prove. As a result, false positives often lead to compliant and effective products being scrapped unnecessarily.
Protect Your Operation With an Isolator for Sterility Testing

Regulatory guidance on isolators:
- EU GMP Annex 1 section 10.6 states:
“The sterility test should be performed under aseptic conditions”
- The FDA’s Guidance for Industry for Sterile Drug Products Produced by Aseptic Processing takes things one step further and states in section XI:
“The use of isolators for sterility testing helps minimise the chance of a false positive test result”
- According to EU GMP Annex 1 an isolator is:
“An enclosure capable of being subject to reproducible interior bio-decontamination, with an internal work zone meeting Grade A conditions that provides uncompromised, continuous isolation of its interior from the external environment”

Isolators have a number of features to protectaseptic processes including:
- Air tight/inflatable seals on all doors
- Interlocks to prevent doors being opened once aseptic conditions have been achieved (following bio-decontamination)
- Unidirectional airflow between 0.36-0.45 m/s (to comply with EU GMP Annex 1 guidance values)
- HEPA filters to cleanup incoming air
- Pressure differentials, pressure testing of enclosure and gloves separately
- Environmental monitoring systems to confirm that the environment remains aseptic during use
- Airflow and pressure alarms
- Hydrogen peroxide vapour bio-decontamination system achieving a 6-log sporicidal kill* to eliminate contamination on the enclosure surfaces and incoming materials needed for the testing process
*When used according to the label instructions, Ecolab Bioquell systems utilize Bioquell Hydrogen Peroxide Sterilant-AQ (EPA Registration Number:1677-277)
Benefits of Isolators
Primarily, isolators reduce the risk of false positives occurring during sterility testing which can save manufacturers of sterile products millions of dollars by minimising unnecessary scrapping of product.
As a secondary benefit, isolators can also provide substantial savings to operating costs.
Unlike a Biological Safety Cabinet (BSC) / Laminar Air Flow (LAF), which must be situated in a Grade B cleanroom, isolators can be situated in a lower Grade C/D cleanroom. This can result in substantial savings from:
- Energy bills from lower capacity HVAC system
- Reduced labour resource (for cleaning and disinfection)
- Less maintenance of the cleanroom
- Reduced cleaning and disinfection consumable costs
- Less gowning requirements
- Increased efficiency of operators as lower gowning means they can work for longer periods
1Costing a Cleanroom Per Square Foot, Cleanroom Technology, 28 February 2018


Comparing Traditional Stainless Steel Isolators to the Bioquell Qube