
Purate™ Chemistry
Product Details
How does ClO2 compare to other technologies?
Chlorine dioxide (ClO2) is a remarkable molecule that performs well when compared to other technologies.
Chlorine dioxide vs. Chlorine
Safety concerns with chlorine gas have prompted many to switch to alternate products such as chlorine dioxide (ClO2).
Chlorine, as chlorine gas or sodium hypochlorite has been used for years as a pre-oxidant and primary disinfectant. However, its use continues to decrease because of the problems associated with the formation of by-products of concern. Chlorine is a weaker antimicrobial than ClO2, particularly at pH levels above 7. Although chlorine is generally less expensive per unit weight, the overall treatment cost with ClO2 is often less because of its increased efficacy. Chlorine can cause odour problems, but ClO2 can solve taste and odour problems.
Because ClO2 produced less by-products of concern, it is often used at the beginning of the water plant as a pre-oxidant, often for control of Fe and Mn.
Chlorine dioxide vs. Ozone
The strong oxidizing potential of ozone leads to the formation of bromate, a regulated carcinogen in drinking water.
Ozone is a stronger antimicrobial than ClO2. However, in water sources containing bromide, the allowable dose of ozone can be severely limited by the bromate limits. Because of the high reactivity of ozone, a residual is short-lived and difficult to measure. Plus operating and maintenance costs for ozone systems are high.
Chlorine dioxide systems are much less expensive to install.
Chlorine dioxide vs. UV
UV final treatment combined with chlorine dioxide (ClO2) pre-treatment can be a very effective water treatment solution.
Both UV and ClO2 require short contact times for inactivation of microorganism and are unaffected by ammonia in the water. UV systems are costly to install, operate and maintain. A back-up power supply is often specified for a UV system which adds significant cost to the system because of the high power demand.
Because UV treatment provides no residual disinfectant, bacteriological testing is require to determine the effectiveness, taking more than 24 hours to complete.
Name: Chlorine Dioxide
Synonyms: "Chlo-2", chlorine oxide, chlorine peroxide
Formula: ClO2
Molecular Weight: 67.4518
Cas No. 10049-04-4
Bond Angle: 117.5°
Bond Length: 0.147 nm
Dipole Moment:5.95x10-30 C*m
Acentric Factor: 0.35638
Structure:


| Physical State Properties | Thermodynamic Properties |
|---|---|
| Appearance: Yellow-green to orange-red gas, Red crystalline solid. | Heat of Formation: 24.50 kcal/gm-mole |
| Usual Shipping State: Generated on-site; shipping is not permitted | Gibbs Energy of Formation: 28.80 kcal/gm-mole |
| Melting/Freezing Point: -59.5°C (-75.1°F) | Ideal Gas Entropy: 0.257kJ/gm-mole K |
| Boiling Point: 10.9°C (51.6°F) at 760 mmHg 9.9°C (49.8°F) at 731 mmHg |
Net Heat of Combustion (gas): -24.50 kcal/Gm-mole |
| Critical Temperature: 192°C (377.6°F) | Heat of Solution in Water: 6.6 kcal/gm-mole |
| Critical Pressure: 8621.6kPa (1250.6 psia) | Liquid Molar Volume: 4.1852x10-2 m3/kmol |
| Triple Point Temperature: -59.5°C (-75.1°F) |
Triple Point Pressure: 1.2544 kPa (9.4 mmHg abs) |
| Densities | Temperature/Dependent Properties |
|---|---|
| Liquid: 1.773 g/mL at -55°C 1.640 g/mL at 0°C 1.614 g/mL at 10°C |
Gas Heat Capacity: 0.0408 kJ/(gm-mole K) at 0°C 0.0417 kJ/(gm-mole K) at 20°C 0.0425 kJ/(gm-mole K) at 40°C |
| Gas: 3.09 g/l at 11°C | Heat of Vaporization: 26.937 kJ/gm-mole at 0°C 25.825 kJ/gm-mole at 20°C 24.629 kJ/gm-mole at 40°C |
For all of the chemists and engineers in the room, back to the periodic table of elements and stoichiometry.
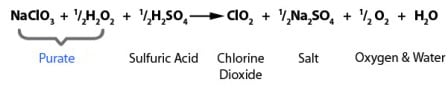
PURATE - High Efficiency
PURATE ClO2 chemistry highlights the reduction of sodium chlorate with hydrogen peroxide under acidic conditions to produce chlorine dioxide (ClO2). It’s made easy with our pre-blended PURATE solution.
Competitors - Low Efficiency
Some chlorite-based generators can achieve 95% efficiency when properly calibrated. However, three-chemical generators (sodium chlorite + hypochlorite + HCl) quickly lose performance and require frequent adjustments and calibrations. As a result, they typically operate with lower efficiency.
One particular chlorite technology (sodium chlorite + HCl) can operate with high efficiency but with a low yield. Inherent in the chemistry is the need to use 5 molecules of sodium chlorite to produce 4 molecules of ClO2, making 80% the maximum possible yield. Because of low yields, this technology is economically limited to small accounts, typically less than 1 kg/hr.
![]()
Chlorine Dioxide generated by PURATE is globally accepted, CE certified (equipment only), EPA registered, FDA approved, BfR approved, Kosher (OU) certified and NSF 60 certified (equipment / precursor).
PURATE and sulfuric acid are NOT covered chemicals under Process Safety Management (PSM). Even though ClO2 is a covered chemical, it is generated on site and used immediately, so the threshold quantity (TQ) is never even approached.
ClO2 produced from PURATE avoids all of the PSM and Risk Management Programme (RMP) requirements and associated costs.


